
Research
Three-dimensional nanoporous graphene
Three-dimensional (3D) nanoporous graphene with preserved 2D Dirac electronic characters was successfully synthesized. The nanoporous graphene is constructed by a single layer graphene sheet that is continuously inter-connected to form a complex 3D network structure. This free-standing nanoporous graphene with an excellent crystallinity possesses high electron mobility, holding great promise for the applications in electronic, optic and energy related devices. The nanoporous graphene were grown by a nanoporous metal based chemical vapor deposition (CVD) method in Figure (a). The overall morphology of the nanoporous graphene in Figure (b) shows a 20 um thick free-standing bulk sheet. Although the 3D nanoporous graphene has a complex structure, it is demonstrated to be ~5000 cm2/Vs in electron mobility and a mass-less Dirac cone system. As the conventional transistor requires electron mobility of 200 cm2/Vs, it is greatly expected that this nanoporous graphene will bring new 3D graphene devices.
References
- Gap opening in double side highly hydrogenated free standing graphene, Nano lett. 2022, 7, 2971-2977.
- Dirac Fermion kinetics in three-dimensionally curved graphene, Adv. Mater., 2020, 32, 2005838.
- Extraordinary tensile strength and ductility of scalable nanoporous graphene, Sci. Adv., 2019, 5, eaat6951.
- Lithium intercalation into bilayer graphene, Nat. Comm., 2019, 10, 275.
- Three-dimensional porous graphene networks expand graphene-based electronic device applications, Phys. Chem. Chem. Phys., 2018, 20, 6024-6033.
- Terahertz and mid-infrared plasmons in three-dimensional nanoporous graphene, Nat. Comm. 2017, 8, 14885.
- Electric Properties of Dirac Fermions Captured into 3D Nanoporous Graphene Networks, Adv. Mater., 2016, 28, 10304-10310.
- Multi-functional porous graphene for high-efficiency steam generation by heat localization, Adv. Mater., 2015, 27, 4302-4307.
- High Quality Three-Dimensional Nanoporous Graphene, Angew. Chem. Int. Ed. 2014, 53 4822−4826. (Hot Paper)
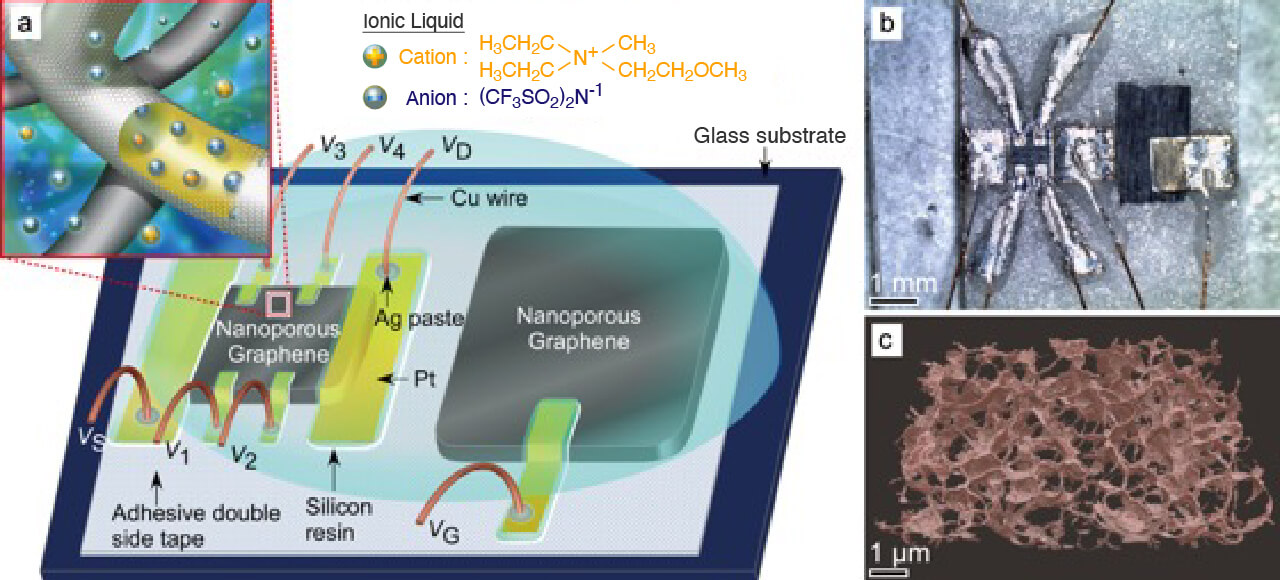
- 3D graphene transistor
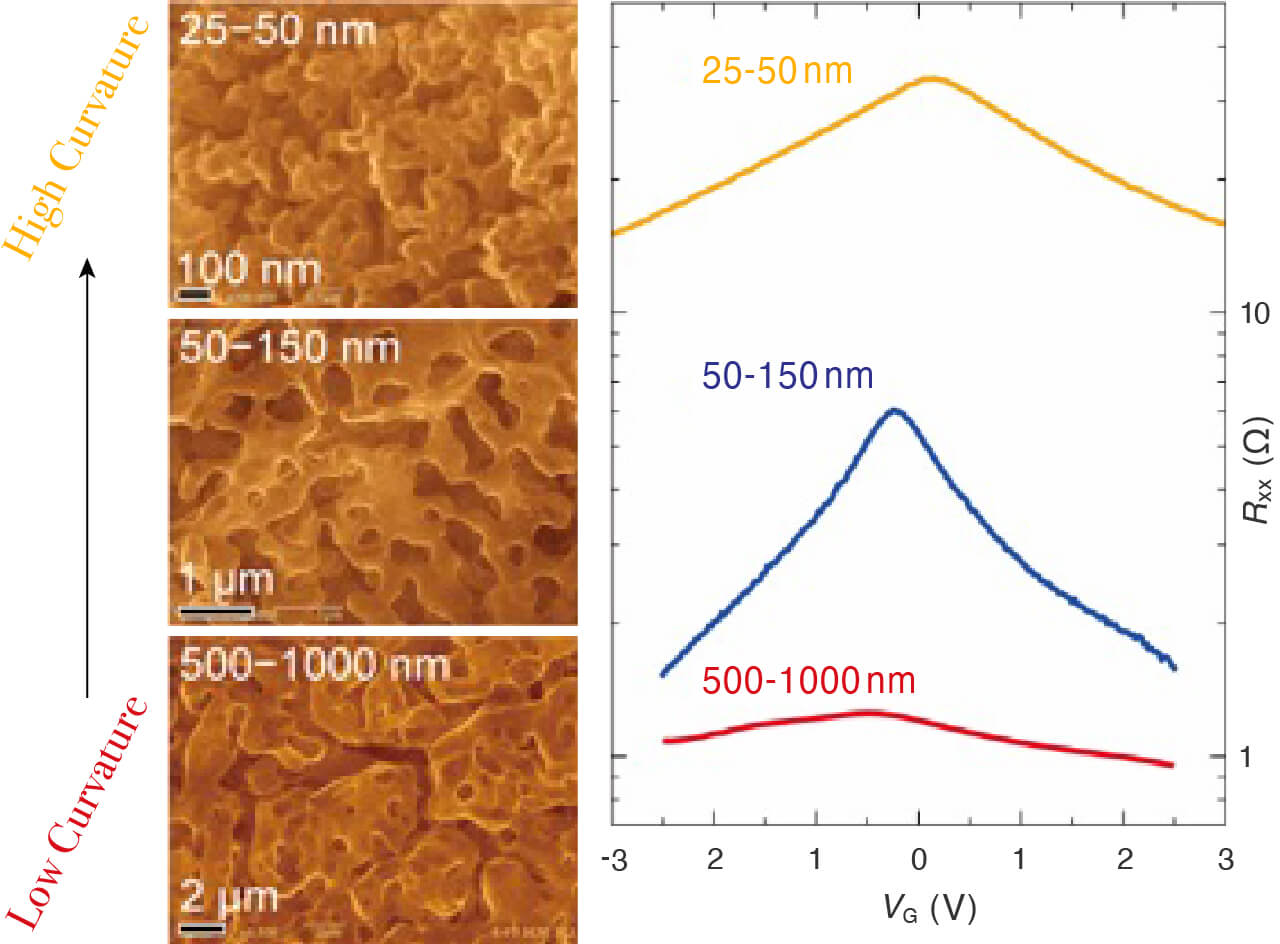
- SEM images and capacitance of 3D graphene with different pore size
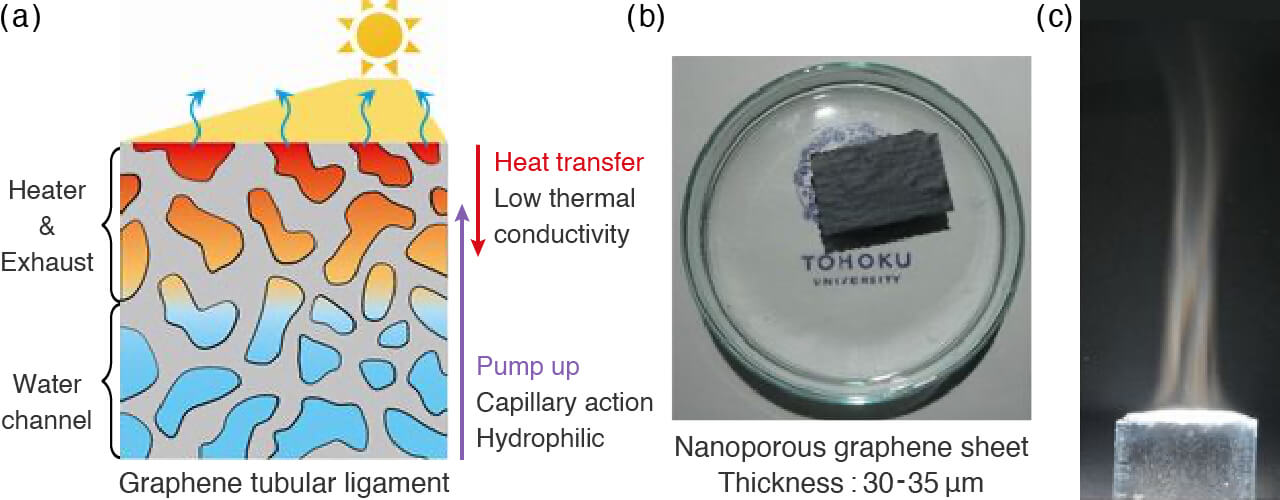
- Steam generation by a thin porous graphene sheet.
Three-dimensional nanoporous graphene for hydrogen evolution
Three-dimentional (3D) nitrogen(N) and sulfur(S) co-doped nanoporous graphene with preserved high conductivity was successfully synthesized. The 3D NS-doped nanoporous graphene is constructed by a few layer graphene sheets that are continuously inter-connected to form a complex 3D network structure. Due to the nitrogen and sulfur co-doping and abundant nanoporous structures, the catalysis towards hydrogen evolution reaction has been successfully activated inside and outside the nanoporous graphene at absence of any metal catalysts. This hydrogen harvesting technology with 3D metal-free graphene catalyst shows a great promise for achieving economic hydrogen energy at high efficiency for green energy innovation.
References
- Chemical dopants on edge of holey graphene accelerate electrochemical hydrogen evolution reaction, Adv. Sci., 2019, 6, 1900119.
- Heavily Doped and Highly Conductive Hierarchical Nanoporous Graphene for Electrochemical Hydrogen Production Angew. Chem. Int. Ed. 2018, 57, 13302-13307.
- Correlation between Chemical Dopants and Topological Defects in Catalytically Active Nanoporous Graphene, Adv. Mater., 2016, 28, 10644-10651.
- Nanoporous Graphene with Single-Atom Nickel Dopants: An Efficient and Stable Catalyst for Electrochemical Hydrogen Production Angew. Chem. Int. Ed. 2015, 54, 14031−14035. (Hot Paper)
- High Catalytic Activity of Nitrogen and Sulfur Co-Doped Nanoporous Graphene in the Hydrogen Evolution Reaction Angew. Chem. Int. Ed. 2015, 54, 2131-2136.
- Bicontinuous Nanoporous N-doped Graphene for Oxygen Reduction Reaction Adv. Mater. 2014, 26, 4145−4150.
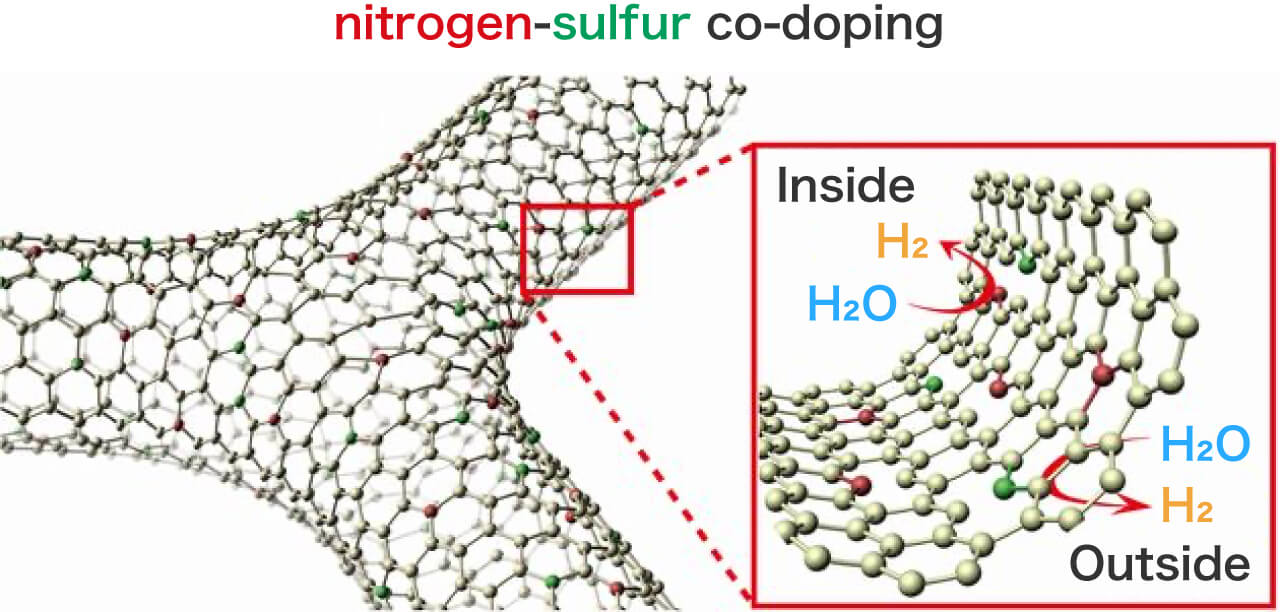
- Figure Metal-free chemically doped three dimensional graphene electrode for hydrogen evolution. References:
Acceleration of electrochemical CO2 reduction by catalyst supports
Accelerating the CO2-recycling process is crucial for preventing global warming. Electrochemical reduction allows the efficient conversion of CO2 into useful chemical compounds with catalysts. During the electrolytic synthesis of CO2, an increase in voltage accelerates the synthesis of the target product and enhances byproduct formation. Thus, we are looking for new mechanisms that allow us to accelerate the production rate (high speed) and prevent byproduct formation (high selectivity) simultaneously.
References
- Acceleration of Electrochemical CO2 Reduction to Formate at the Sn/Reduced Graphene Oxide Interface ACS Catal., 2021, 11, 3310-3318
- Polyethylene Glycol Covered Sn Catalysts Accelerate the Formation Rate of Formate by Carbon Dioxide Reduction ACS Catal., 2021, 11, 9962-9969
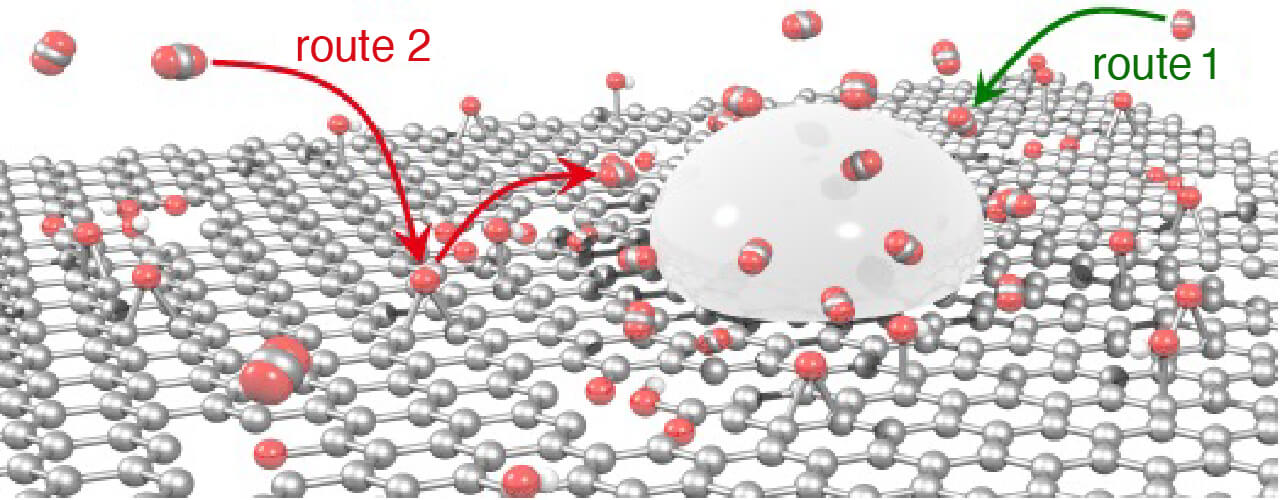
- New CO2 supply route 2 accelerates electrochemical CO2 reduction
Corrosion resistant non-noble metal catalyst
The dream of hydrogen-powered cars has excited many people as a solution for the huge amount of carbon dioxide fossil-fuel burning vehicles emit into the atmosphere daily. However, the production of hydrogen gas has been slowed by the lack of cheap catalysts required to split water efficiently. In this process, hydrogen nuclei, called protons, need to combine to form hydrogen gas, H2. Nickel and Ni-based alloy are seen as promising cheap alternatives to platinum, but these metals corrode easily when exposed to the acidic conditions of the reaction. One solution is to use graphene, a single sheet of carbon atoms arranged in a honeycomb lattice, to protect the catalyst. Thus, we are challenging to employ the graphene covering non-noble electrodes or other new catalysts in PEM-type water electrolyzers for practical applications.
References
- Catalytic Activity of Graphene-Covered Non-Noble Metals Governed by Proton Penetration in Electrochemical Hydrogen Evolution Reaction Nat. Comm., 2021, 12, 203
- Corrosion-resistant non-noble metal electrodes for PEM-type water electrolyzer Int. J. Hydrogen Energy, 2021, 46, 38603-38611.
- Effect of Graphene Encapsulation of NiMo Alloys on Oxygen Evolution Reaction, ACS Catal., 2020, 10, 792-799.
- Graphene Layer Encapsulation of Non-Noble Metal Nanoparticles as Acid-Stable Hydrogen Evolution Catalysts, ACS Energy Lett., 2018, 3, 1539-1544.
- Cooperation between holey graphene and NiMo alloy for hydrogen evolution in acidic electrolyte, ACS Catal. 2018, 8, 3579-3586.
- News release: Giving the Hydrogen Economy an Acid Test
- Graphene covering Ni electrode after HER tests.
- A full cell experiment
Metal-air batteries
High capacity metal-air (Li, Zn, K etc) battery is a new type of secondary battery which has attracted great attention in recent years because of the high theoretical capacity and energy density. However, the practical implementation of the new battery is limited by the low cycling stability and poor energy efficiency. To solve these challenging issues, developing new cathode materials with high electrochemical stability and low charge/discharge overpotentials is a crucial topic to realize carbon neutral society.
References
- Inhibiting Surface Diffusion to Synthesize Three-Dimensional Bicontinuous Nanoporous N-Doped Carbon for Boosting Oxygen Reduction Reaction in Flexible All-Solid-State Al-Air Batteries, Adv. Funct. Mater., 2021, 31, 2103632 (Back Cover).
- Anchoring Single-Atom Mo and N on Edge-Rich Nanoporous Holey Graphene as Bifunctional Air Electrode in Zn−Air Batteries Appl. Catal. B: Environmental, 2020, 276, 119172.
- Building a Reactive Armor Using S-Doped Graphene for Protecting Potassium Metal Anodes from Oxygen Crossover in K–O2 Batteries, ACS Energy Lett., 2020, 5, 1788-1793.
- Metal and Nonmetal Codoped 3D Nanoporous Graphene for Efficient Bifunctional Electrocatalysis and Rechargeable Zn-Air Batteries, Adv. Mater., 2019, 31, 1900843.
- 3D nanoporous nitrogen-doped graphene with encapsulated RuO2 nanoparticles for Li-O2 battery, Adv. Mater., 2015, 27, 6137-6143.
- Effect of chemical doping on cathodic performance of bicontinuous nanoporous graphene for Li-O2 batteries, Adv. Energy Mater. 2016, 6, 1501870.
- Full Performance Nanoporous Graphene Based Li-O2 Batteries Through Solution Phase Oxygen Reduction and Redox-Additive Mediated Li2O2 Oxidation, Adv. Ener. Mater. 2017, 7, 1601933.
- News release: Development of high-performance rechargeable Li-air battery
- News release: Nanoparticles of ruthenium oxide trapped between atom-thin layers of carbon boost the performance of lithium–oxygen batteries
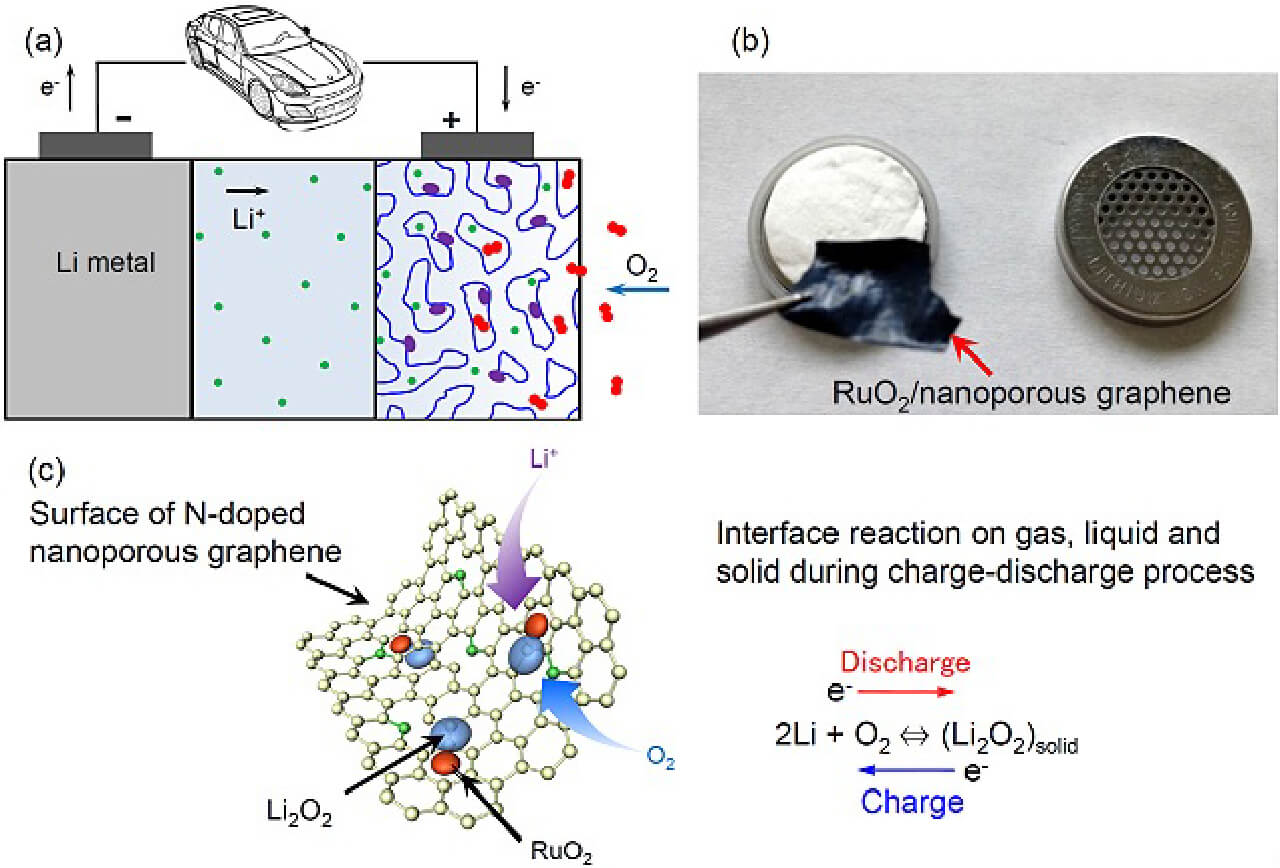
- Li-O2 battery and mechanism of the electrode reactions
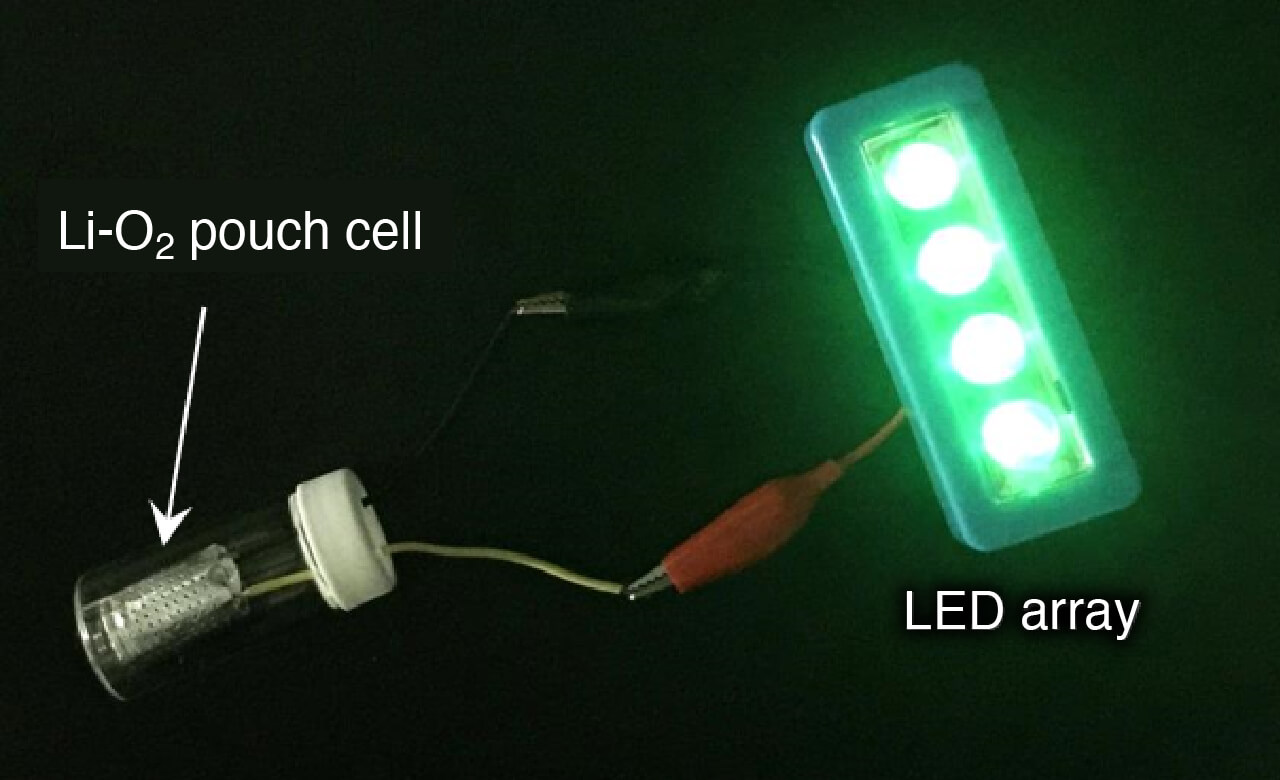
- Demonstration of Li-O2 battery (green LED)
The mathematics of repulsion for new graphene catalysts
Changing the geometry of three-dimensional (3D) graphene, which is made of networks of carbon atoms, by adding chemicals or introducing topological defects, can improve its catalytic properties. The new mathematical model, called standard realization with repulsive interaction (SRRI), reveals the relationship between these changes and the properties that arise from them. The mathematical model can be used as an effective pre-screening tool for exploring new 2D and 3D carbon materials for unique properties. We are looking for a new carbon network for physics and chemistry.
References
- Geometric model of 3D curved graphene with chemical dopants Carbon, 2021, 173, 403-409.
- News release: The mathematics of repulsion for new graphene catalysts
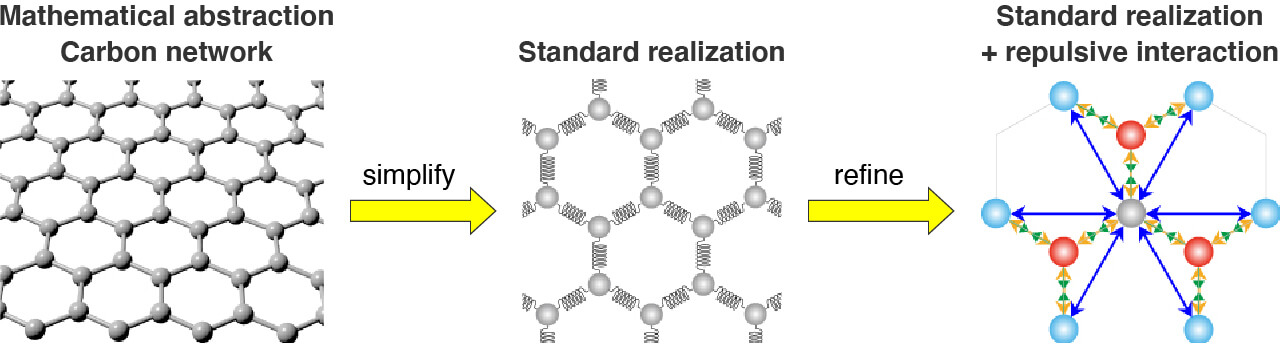
- Simplification of carbon network and mathematical determination of geometry. Mathematical abstraction from carbon network to mathematical modeling for the standard realization and the standard realization with repulsive interaction.